Clinical Job at Novo Nordisk in Bangalore for BDS / MDS Graduates
Looking to advance your career in global clinical development? This clinical job opportunity at Novo Nordisk offers healthcare professionals a chance to work at the forefront of dose surveillance and patient safety. Based in Bangalore, this role is ideal for candidates seeking impactful Novo Nordisk career growth and high-value jobs in Bangalore within clinical research and monitoring.
Job Details:
- Job Title: Dose Surveillance Advisor
- Category: Clinical Development
- Department: Centralized Monitoring Unit, Bangalore
- Company: Novo Nordisk Global Business Services (GBS), India
- Location: Bangalore, Karnataka, IN
About the Company
Novo Nordisk is a global healthcare company committed to driving change in diabetes, obesity, and serious chronic diseases. With a strong focus on innovation, research excellence, and patient safety, a Novo Nordisk career offers professionals the opportunity to work in high-impact global clinical programs. The organization’s Global Business Services (GBS) unit in India plays a vital role in supporting worldwide clinical development initiatives, making it a preferred destination for professionals seeking long-term growth and meaningful jobs in Bangalore.
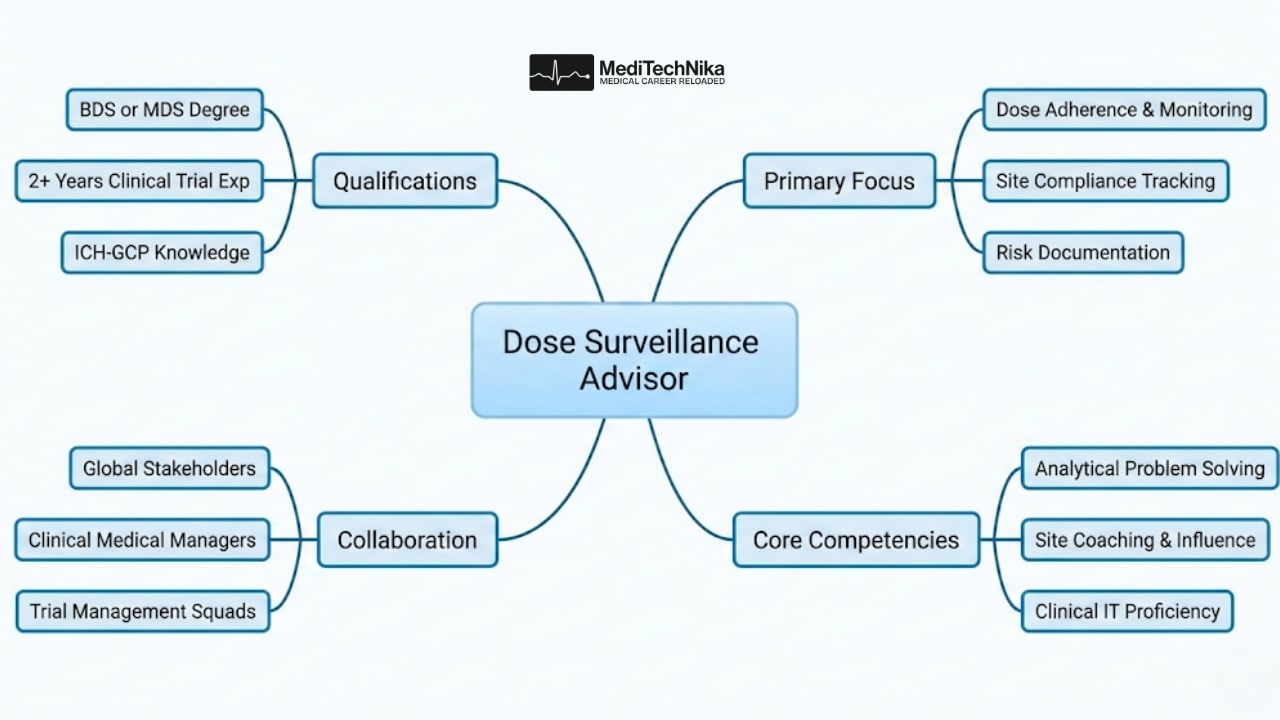
Qualifications
Education
Graduate degree in BDS/MDS with a strong understanding of clinical trial conduct and ICH-GCP.
Experience
- Minimum 2 years in clinical trials within pharma/biotech/CRO/hospital settings (mandatory).
- Strong working knowledge of trial data, systems, and global clinical operations.
- Experience collaborating with diverse global stakeholders.
Skills & Competencies
- Excellent communication and relationship-building skills.
- Ability to independently solve complex issues in evolving environments.
- Strong analytical mindset and familiarity with clinical IT systems.
- High motivation, ownership, and a continuous improvement mindset.
- Ability to coach, influence, and manage stakeholders effectively.
Job Description
As a Dose Surveillance Advisor, you will play a key role in supporting dose surveillance and retention efforts across global clinical trials.
Key Responsibilities
- Overseeing adherence to prescribed dose status across assigned trial sites in collaboration with Clinical Medical Managers (CMMs) and Medical Advisors (MS).
- Proactively identifying prescribed vs. expected dose discrepancies, engaging with sites, and driving clear, traceable follow-ups.
- Documenting dose-related operational risks in applicable tools and ensuring inspection-ready records.
- Supporting site engagement through clear communication, data insights, and timely escalation of issues.
- Executing routine and ad-hoc dose surveillance reviews in accordance with protocol requirements.
- Collaborating closely with Trial Managers on dose- and retention-related matters, contributing to site performance and trial quality.
- Coaching site staff to strengthen dose compliance practices, documentation quality, and protocol adherence.
- Communicating findings to trial squads and medical partners to support informed decision-making.
- Contributing to continuous improvement initiatives and helping shape this evolving surveillance function.