Build Your Career with an Analyst Job at Merck in Bangalore | Apply Now
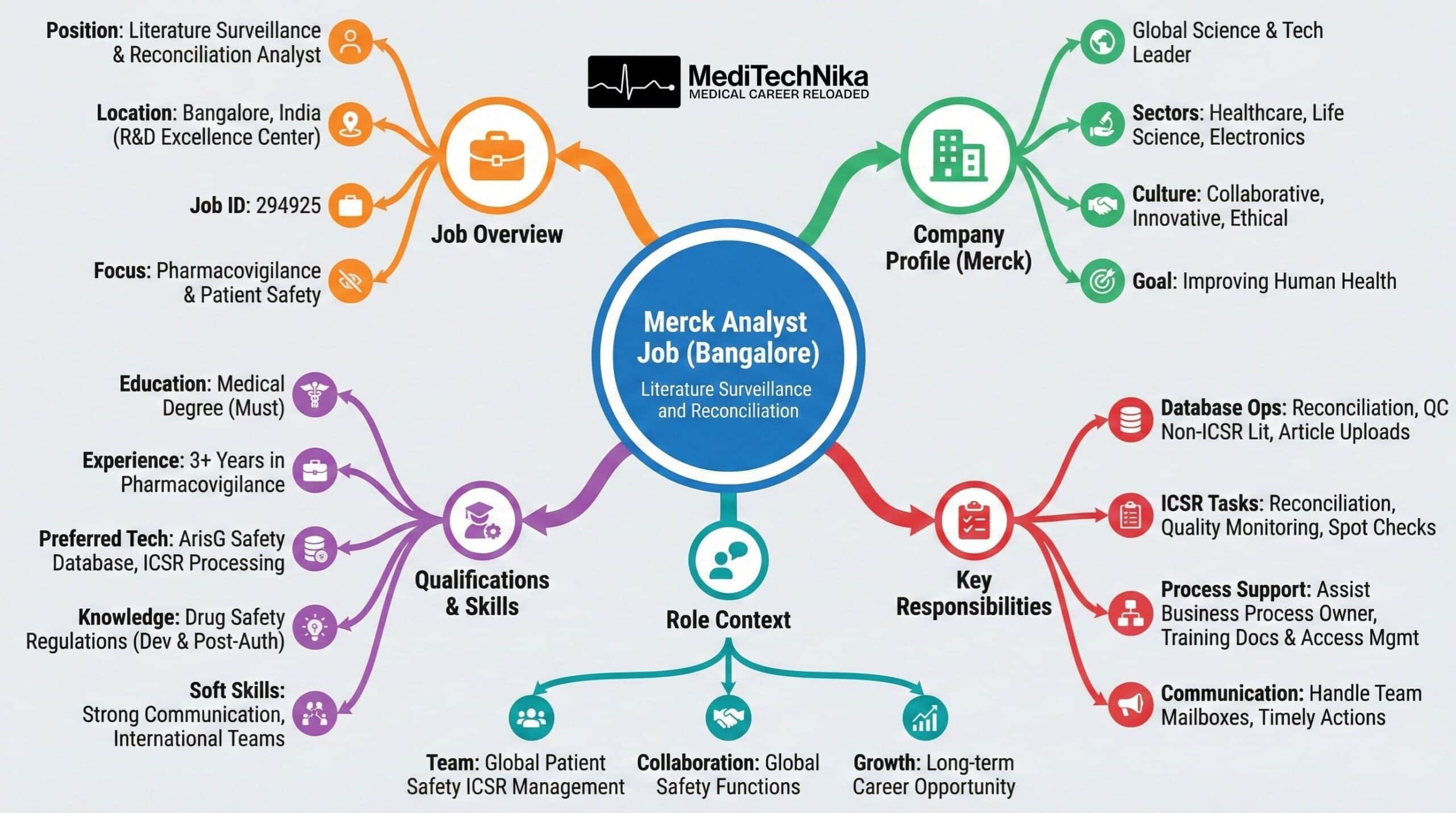
The analyst job at Merck presents a valuable opportunity for professionals looking to grow in pharmacovigilance and patient safety. This role, based in Bangalore, supports global literature surveillance and ICSR reconciliation activities while offering exposure to international teams. For candidates exploring Merck careers, this position combines scientific expertise, regulatory compliance, and impactful medical jobs in a global organization.
Job Details:
- Job Position: Literature Surveillance and Reconciliation Analyst
- Location: Bangalore, Karnataka, India
- Job ID: 294925
About the Company:
Merck is a global science and technology leader with a strong presence across Healthcare, Life Science, and Electronics. The company is driven by innovation, research excellence, and a deep commitment to improving human health and quality of life. Through its collaborative work culture, Merck empowers employees to contribute to impactful medical jobs while working on solutions that address real-world challenges. With diverse global teams and a focus on ethical science, Merck careers offer professionals the opportunity to grow, learn, and make a meaningful difference worldwide.
Qualifications:
- Medical degree with at least 3 years of experience in the pharmacovigilance environment.
- Prior experience in literature safety surveillance and ICSR reconciliation is preferred for this analyst job.
- Sound understanding of regulations related to drug safety during development and post-authorization.
- Ideally, ICSR case processing experience in the ArisG safety database environment.
- Strong communication skills and experience working in international and matrix teams, aligned with global Merck careers standards.
Job Description:
This analyst job is part of the Global Patient Safety ICSR Management team at the R&D Excellence Center in Bangalore. The role focuses on supporting literature surveillance processes, reconciliation activities, and quality monitoring while collaborating with global safety functions. It is well-suited for professionals seeking long-term growth through Merck careers and specialized medical jobs.
Key Responsibilities:
- To support the business process owner for the literature surveillance process.
- To support training documentation and user access management in the literature surveillance and reconciliation management databases.
- Perform operational management activities in the literature surveillance database, including reconciliation, QC of non-ICSR literature, and article uploads.
- Perform ICSR reconciliation, quality monitoring, and spot checks of safety information with relevant functions.
- Handle team mailboxes and ensure that all received emails are actioned on time as part of this analyst’s job.