BDS Job at Syneos Health | Jobs in Gurugram & Hyderabad
Syneos Health is offering an exciting BDS job opportunity for dental professionals seeking to transition into pharmacovigilance and drug safety roles. This role, Safety & PV Specialist, is part of a global Syneos Health career pathway and is suitable for BDS graduates looking for non-clinical dental jobs in Gurugram and Syneos Health Hyderabad, with strong exposure to ICSR processing, regulatory compliance, and global safety operations.
Job Details:
- Job Role: Safety & PV Specialist I
- Qualification: BDS
- Experience: Minimum 2 Years
- Location: Gurugram/Hyderabad
About the Company
Syneos Health is a leading, fully integrated biopharmaceutical solutions organization built to accelerate customer success. As part of a global Syneos Health career, employees work within a Clinical Development model that places the customer and patient at the center of everything the organization does. Syneos Health offers diverse opportunities, including non-clinical dental jobs for healthcare professionals such as BDS graduates. Syneos Health is committed to professional growth, diversity, and its Total Self culture, creating an inclusive environment where employees can build long-term careers, including roles based in Syneos Health Hyderabad and Gurugram.
Qualifications
- Educational Qualification: BDS
- Experience: Minimum 2 years
- Preferred experience in CT case processing or literature case processing
- Strong pharmacovigilance and safety reporting knowledge
- Knowledge of safety database systems and medical terminology
- Understanding of clinical trial phases II–IV and post-marketing safety requirements
- Knowledge of ICH GCP, GVP, and safety regulations
- Proficiency in Microsoft Word, Excel, PowerPoint, Visio, Outlook, and collaboration tools
- Ability to work independently and as part of a team
- Excellent written and verbal communication skills
- Strong organizational skills and attention to detail
Job Description
This BDS job at Syneos Health involves working as a Safety & PV Specialist I, supporting pharmacovigilance activities across clinical trials and post-marketing programs. The role focuses on ICSR processing, safety database entry, MedDRA coding, literature screening, and regulatory safety reporting, making it an excellent career option for BDS professionals seeking alternative dental jobs outside clinical practice.
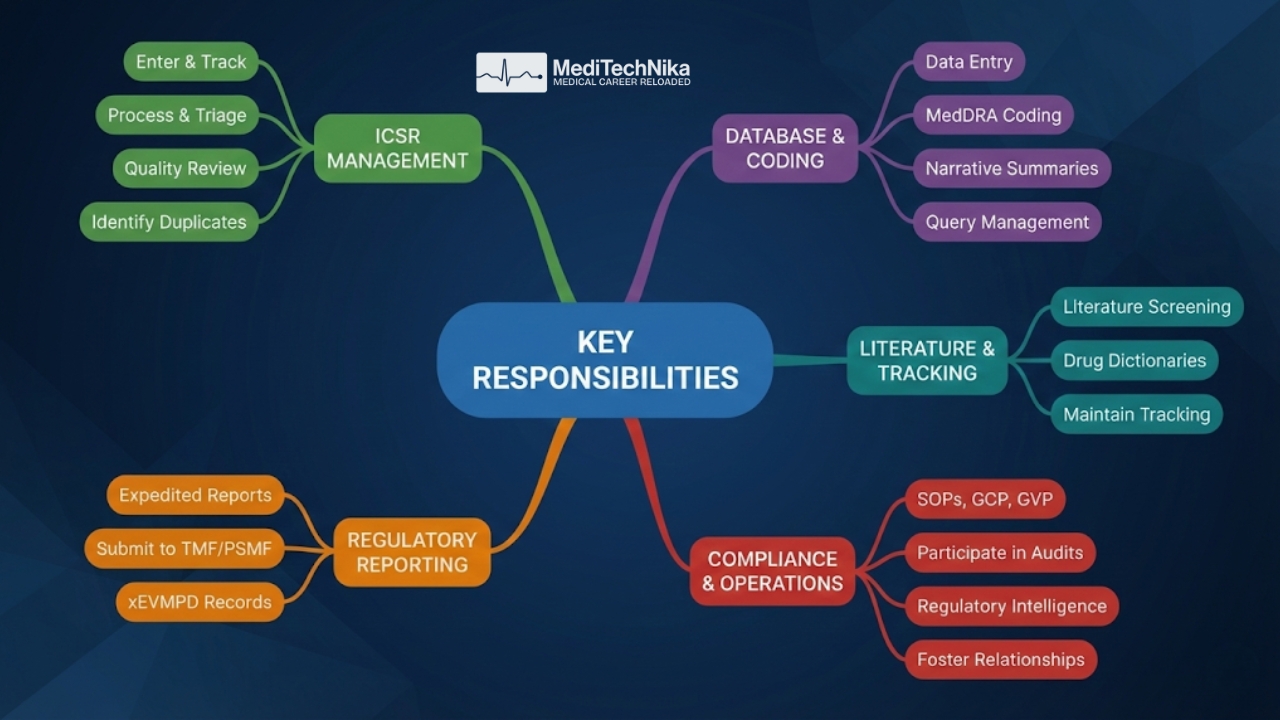
Key Responsibilities
- Enter information into PVG quality and tracking systems for receipt and tracking of ICSRs
- Assist in the processing of ICSRs according to SOPs and project/program-specific safety plans
- Triage ICSRs and evaluate data for completeness, accuracy, and regulatory reportability
- Enter data into safety databases and code events, medical history, concomitant medications, and tests
- Compile complete narrative summaries for safety cases
- Identify information to be queried and follow up until queries are resolved
- Assist in the generation of timely and accurate expedited safety reports
- Maintain safety tracking for assigned activities
- Perform literature screening and review for safety and drug coding
- Maintain drug dictionaries and perform MedDRA coding
- Validate and submit xEVMPD product records with appropriate MedDRA coding
- Perform manual recoding of unrecoded product and substance terms
- Identify and manage duplicate ICSRs
- Support SPOR / IDMP-related activities
- Conduct a quality review of ICSRs
- Ensure submission of documents to the TMF and Pharmacovigilance System Master File
- Maintain compliance with SOPs, GCP, ICH guidelines, GVP, and global safety regulations
- Foster professional relationships with internal and external teams
- Participate in audits as required
- Apply regulatory intelligence across all safety reporting activities